Accelerate Drug Controlled Release Studies with Mott’s Advanced Diffusion Technology
Accelerating Drug Controlled Release Studies with Mott’s Advanced Diffusion Technology
Device diffusion testing is a critical step that can delay the time for an implantable drug delivery device to get through clinical trials, regulatory hurdles, and ultimately, to market. Mott Corporation has developed a methodology for conducting controlled drug release studies that significantly reduces this time. By combining porous membrane media with established surrogate drug suspensions, Mott conducts rapid in vitro studies. These studies can be run in as little as one day, accurately extrapolating the controlled release of therapeutics over months. This fast and accurate approach narrows the scope of in vitro testing, dramatically reducing the cost and time associated with animal trials.
Controlled Release Drug Diffusion Studies
Device diffusion testing is a critical step that can delay the time for an implantable drug delivery device to get through clinical trials, regulatory hurdles, and ultimately, to market. Mott Corporation has developed a methodology for conducting controlled drug release studies that significantly reduces this time. By combining porous membrane media with established surrogate drug suspensions, Mott conducts rapid in vitro studies. These studies can be run in as little as one day, accurately extrapolating the controlled release of therapeutics over months. This fast and accurate approach narrows the scope of in vitro testing, dramatically reducing the cost and time associated with animal trials.
The Importance of Controlled Drug Release in Medical Devices
Controlled drug release, or drug controlled release, is a method used to deliver medications at a predetermined rate, for a specified period, to achieve a therapeutic effect. This technique is crucial for maintaining the desired drug concentration in the body, minimizing side effects, and improving patient compliance. The development of controlled release systems is a complex process that requires thorough testing to ensure efficacy and safety.
Mott’s Approach to Drug Controlled Release Studies
Mott’s controlled release studies guide the design criteria for next-generation drug delivery technology. These diffusion studies provide reliable early assessment of drug diffusion rates and insights into the form, fit, and function of a device.
The Model
Mott uses a model system to conduct drug diffusion studies. This system involves a reservoir containing the surrogate drug suspension, which is fitted with various diffusion membranes to be evaluated for their diffusion rates within a bath of interstitial fluid surrogate, mimicking the in vivo implant environments of an implanted device.
Figure 1: General Layout of the Assemblies for Drug Diffusion Studies
This setup allows Mott to simulate the conditions within the human body, providing accurate and reliable data on how the drug will diffuse over time.
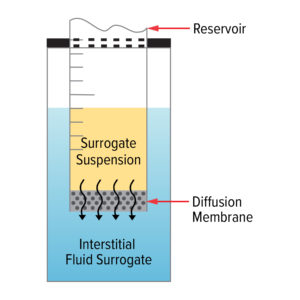
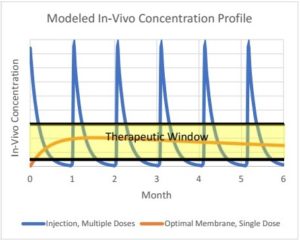
Figure 2: Model Data Derived from In Vitro Diffusion Studies
The data from these studies helps to extrapolate the desired membrane characteristics, comparing them to multiple dose injections to determine their effectiveness in drug controlled release.
Performance Indices and Results
Mott has tested diffusion permeability for durations of 1-day, 5-day, and 50-day periods using its porous membrane technology in conjunction with surrogate media. Studies demonstrated that the rapid 1-day model produces results for various drug compounds within 5% of the empirical data models. This high level of accuracy in a short period allows for faster decision-making and development of drug delivery devices.
Benefits of Mott’s Drug Diffusion Testing Model
Mott offers a fast and reliable drug diffusion testing model that illustrates the effectiveness of rapid in vitro testing. The diffusion study yields a diffusion constant, which correlates to element flow rate and geometry. By establishing a relationship between flow rate and diffusion constant, Mott can determine upper and lower limits on porosity for the application. This delivers critical guidance to the feasibility and design of a device.
Quantitatively, using only one day of data can extrapolate an accurate diffusion constant very close to the 50-day data set. Mott’s ability to conduct in vitro testing reduces the need to test multiple iterations of diffusion technology in vivo. Mott scientists and engineers test Mott’s clinically proven porous membrane guided by therapeutic delivery requirements to identify the most suitable material for the controlled release of your therapeutic.
For more information or to discuss how we can help you with controlled drug release studies, contact us today.